RESEARCH OVERVIEW
|
Our lab is interested in a biological process called autophagy, which is utilized by the cells to perform house-keeping function at basal level or to eliminate toxic materials during stress conditions. Autophagy is an intracellular degradation process for the removal of unwanted macromolecules, damaged organelles and invading pathogens from the cells. During starvation, it also recycles bulk cytoplasmic components to provide inputs to cellular metabolism for generating energy and building new proteins.
|
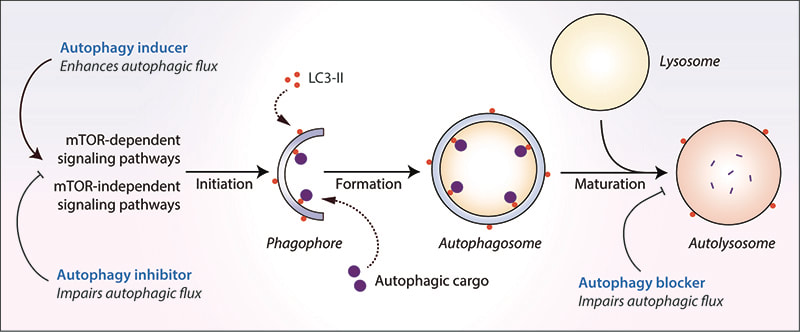
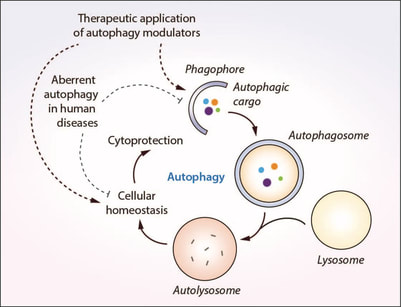
Cells engulf autophagic cargo inside double-membrane vesicles called autophagosomes that are formed from isolation membranes called phagophores. Autophagosomes then fuse with the lysosomes to form the degradative autolysosomes where the cargo is degraded by the lysosomal enzymes. This dynamic process encompassing fusion of vesicular compartments and degradation of cargo is referred to as autophagic flux. Autophagy is classically regulated by the mechanistic target of rapamycin (mTOR) pathway but can be also controlled by mTOR-independent pathways; both avenues being anemable to chemical perturbations. The role of autophagy in the selective clearance of undesirable materials for replacing old cellular components or to combat stress situations, as well as the bulk degradation of cytoplasmic materials during starvation for energy generation is vital for ensuring cellular and energy homeostasis. This homeostatic function of autophagy is essential for cell survival, organ function and human health. Deregulation of this process leads to accumulation of deleterious autophagic cargo that is harmful to the cells. Dysfunction in autophagy occurs in myriad human diseases where it is implicated in the disease pathology whereas therapeutic modulation of autophagy with small molecules is beneficial in certain contexts.
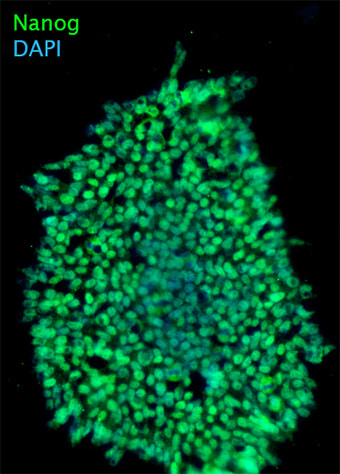
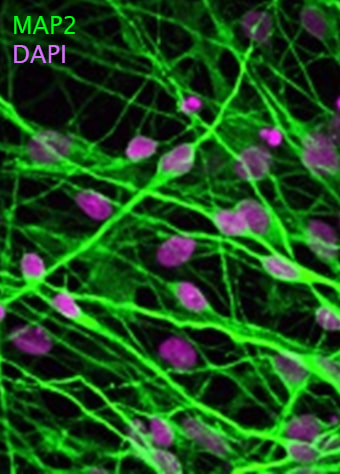
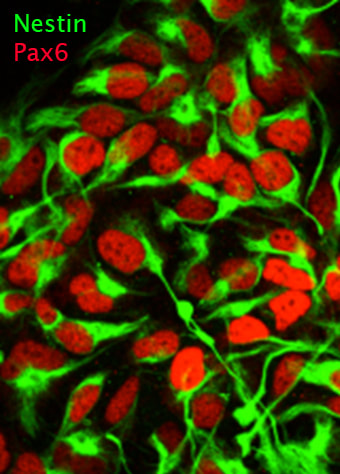
We are utilizing human embryonic stem cells (hESCs) and disease-specific induced pluripotent stem cells (iPSCs) to establish physiological and disease-relevant human cellular platforms for investigating the role, regulation and therapeutic manipulation of autophagy in a manner relevant for human biology. We are differentiating these human pluripotent stem cells into neural precursors, neurons, monocytic cells, macrophages and fibroblasts using established protocols for studying autophagy.
RESEARCH AREAS
|
Autophagy is implicated in diverse human diseases due to its vital role in maintaining cellular homeostasis. Defective autophagy contributes to the cytotoxicity underlying many human pathologies whereas pharmacological upregulation of autophagy is beneficial in various transgenic models including certain neurodegenerative disorders, infectious diseases, liver diseases, myopathies and lysosomal storage diseases.
Research in our lab encompasses the following areas:
|